Thermodynamics
Nowadays, thermodynamics is said to encompass the study of phenomena
in which energy transfer takes place in the form of heat and mechanical
work.
The theoretical basis of thermodynamics is a limited number of principles,
which are generalizations and abstractions of some experimental facts.
The general nature of these principles, which do not contain assumptions
the nature of the forces involved or the microscopic structure of the
studied
systems, makes the methods of thermodynamics applicable to a wide class
of phenomena. Such phenomena are the properties of fluids and solutions,
the balance of states of aggregation, dielectric polarization and magnetization,
electromotive force of galvanic elements, thermal radiation, etc.
Thermodynamics has the historical contribution of personalities who
highlighted processes and phenomena characteristic of the field.
Lavoisier proposes replacing phlogiston with another fluid, caloric.
According to this theory, the amount of caloric is constant in the universe
and it passes from warmer bodies to colder ones. Practically, Lavoisier
said
that in the universe energy is constant and passes from the kinetic state
to
the potential state. Physicist James Clerk Maxwell was one of many who
began to rely on the theory of heat which has to do with the state of
motion
of matter. Maxwell emphasizes and recommends from the book "Heat
as a
mode of motion", by John Tyndall, four provisions for defining heat:
- It is something that can be transferred from one body to another,
according to the second law of thermodynamics.
- It is a measurable quantity and can be treated mathematically.
- It cannot be treated as a material substance because it can be transformed
into something that is not a material substance, for example a mechanical
thing.
- Heat is one of the forms of energy. The quantity that characterizes
the
internal thermal state of a body is called temperature. Temperature is
a
parameter that characterizes "the speed with which the atoms that
make up
a substance move". Since heat (like mechanical work) represents an
amount
of energy transferred between two bodies by certain processes, neither
body
"has" a certain amount of heat, instead a body does have properties
(state functions) such as be the temperature and the internal energy.
Thus, the energy exchanged between particles becomes more and more
disordered. In other words, heat is related to the concept of entropy.
Vector energy
Thermodynamics, its content, along with the content of electronics, are
the consequences of the scientific lie expressed by the definition of
energy:
"Energy is a function of state, of the scalar movement of matter".
In order to move, matter needs an impulse, energy.
Therefore, "energy moves matter to produce energy"!
The interpretation of the thermodynamic and electrical phenomenon,
they copied the lie, without discernment. Temperature (hot, cold), light,
colors do not exist in physical reality, In physical reality there are
only
frequencies, oscillations of vector energy. Temperature (hot, cold), light,
colors exist exclusively in the minds of researchers, in which the sense
organs
have converted external stimuli, oscillations, frequencies (radiations),
into
perceptions. The eye is a complex organ, which evolved through natural
selection specific to animal species, starting with the stigma euglena
viridis.
The visual organ, the eye, converts optical radiation into color images,
which does not exist in reality. The example of such a conversion is
presented by systems with image transmissions. The system transmits
information by emitting a frequency called carrier, modulated by a frequency
called carried, of information. The information is the frequencies emitted
by
the images, which at the reception are demodulated and displayed on a
black
and white screen. If the image frequencies are discriminated by a Bayer
filter,
the image can be displayed in color on the screen. To be noticed, the
real
image and the image displayed on the screen are exclusively frequent,
without light, without colors, these being conversions of the eye.
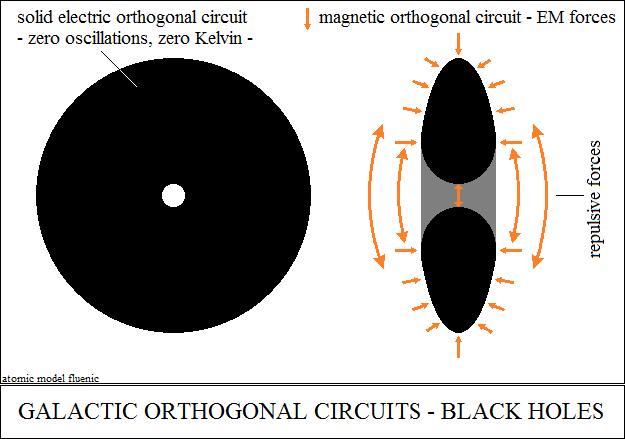
Lavoisier's remark regarding the transition of kinetic energy (hot bodies)
to
potential energy (cold bodies) is found in the internal structure of the
sun.
The "hot" solar sphere is converted by the pressure of the electromagnetic
force, into core, in the electrical circuit, to zero Kelvin. The process
is
exclusively the reduction of vector oscillations to zero. In the universe,
what
we call temperature are the vector oscillations of the frequencies spectrum.
For biological structures, temperature is the interactions of vector oscillations
with frequencies that stimulate or destroy structural bonds (photosynthesis
or fires).
So, biological structures appear, develop or disappear, limited by the
density
of vector oscillations called climate. However, the question remained:
What is energy?
Existence is vector energy and vector energy is the existence.
Matter, atoms, planets, stars, galaxies are structures of vector
energy.
The structures are orthogonally closed vector circuits (electrical
and magnetic),
which oscillate between the kinetic and potential state ("hot and
cold").
Oscillations make up the spectrum of wavelengths (of frequencies) and
implicitly the spectrum of orientation density, of pressure, between the
potential, solid, impenetrable energy state and the kinetic, field state.
Obviously, between these extremes of pressure, there are gas and liquid
states.
The field state, called space, are interactions, connections between solid
states.
It follows that space is the field state of vector energy - vector space.
The gas state is of interest for its properties of elastic expansion,
producing mechanical work: the jet and the expansion in the cylinders.
The phenomenon is the transition of the potential state of the energy
of the
electrical connection circuits in the structure, into the kinetic state
(field, space),
with repulsive forces characteristic of explosive processes.