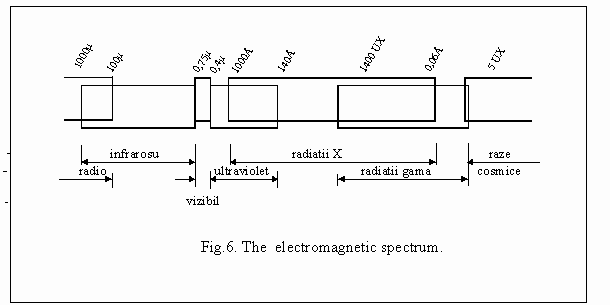
-
The
aquatic environment had probably as primary means of transport the canoe,
a hollowed tree trunk where one or more rower set fig.8. The ship, the
machine of performance, has as propulsion and handling means, of principle
different from the one of the motor car, water being the environment that
scientifical imposed these means. What impression should have made a ship
sent over milleniums, under the glances of the rowers in the canoe? The
first means of transport in the atmosphere illustrate the tendency of
taking over some elements of propulsion, from the means of the other environments,
where they were effective.
-
Actually,
also some types of ships used as means of propulsion the wheel paddles,
taken over from the land environments, so that afterwards the balloon
shall take over for basket the form of ship and the paddles as means of
propulsion fig.9.
-
If
the balloon was the „canoe" of the atmospheric environment, the jet aircraft
is the performance machine. Out of this family of apparatus there was
spotlighted the rocket, that left the atmospheric environment and entered
into ether, in the cosmic space. But, base on the way the other environments
have been conquered, it is easily observed that in fact, the rocket is
the „canoe" of the cosmic space fig.10.
-
Then,
how could look the performance machine of this environment? Out of this
presentation it is spotlighted the idea that only after the solid knowledge
of the cosmic environment, only after the discovery of a phenomenological
and technological principle, that should allow us the „lining" on this
environment, we might build the performance craft.
-
THE
ATOM
-
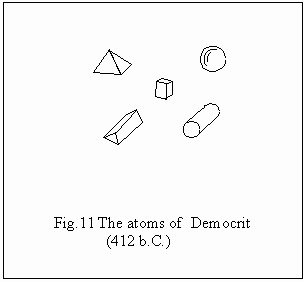
The
notion of atom – indivisible particle – comes from the antiquity, developed
by Democrit of Abdera (420 b.C.). Probably as answer to the idealistic
philosophy. The atoms were in the image of Democrit the constituents of
the universe fig. 11, that moved in the empty vacuum, another notion for
whose control the people shall reach later on to a series of discoveries
regarding the laws of gases, the machine with steam, etc. The atoms of
Democrit had different geometrical forms, by whose combining there resulted
different forms of things, while their movement justifies the idea of
transformation. The corpuscular hypothesis, the atomic one, is reactualized
by Gasendi (1592 – 1655), priest, astronomer, mathematician and philosopher,
who considers the atoms as mass particles, in the inertia state, with
possibilities of movement in the vacuum whose existence has been proved
by Toricelli.
-
THE
STATIC MODEL OF THE ATOM
-
To
the end of the nineteenth century, the avalanche of discoveries released
by theoretical and practical achievements in the field of the electricity,
leads to the need to imagine a model of the atoms´structure.
-
From
the study of catodic radii, one might reach to the conclusion that they
are negative electric charges and the charge unit was named electron,
by Johnston Stoney in 1894. Then, this name goes over to the particle
itself.
-
J.J.
Thomson (1856 – 1940) concluding that the electrons are extracted from
the most different substances, that they are identical and indivisible
particles, consider that they come from the atom, being thus its constituents.
-
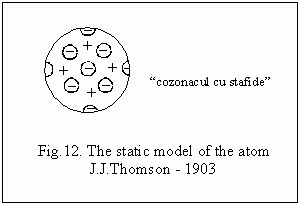
1903
Thomson elaborates the static model of the atom fig. 12, considering the
atom, a spherical mass, uniformely charged with positive charge, in its
interior being the electrons with negative charge, that might be extracted
by irradiation. The negative charges must be equal to the positive charges,
so that the atom is neutral from the electrical point of view.
-
The
static model of the atom couldn´t be adopted by the fact that it doesn´t
explain some phenomenon such as the coexistence of positive and negative
charges, the emission and absorbtion of radiations etc. There have been
proposed some dynamic models, but they kept the same defaults as the static
model. It is discussed, first of all, the problem of the place occupied
by electric charges, if they are distributed in all volume of the atom,
or are localized in a restricted area.
-
THE
PLANETARY MODEL OF THE ATOM
-
In
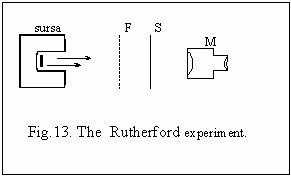
order to give an answer to these questions, it is made a call to the experiment.
Ernest Rutherford designs a parallel beam of radiations alpha – helium
atoms with two positive charges and the mass of 7000 times the mass of
the electron – over a gold film with the thickness of approximately three
atomic layers and studies the form of this beam, beyond the film, by using
the oscialltions produced on a fluorescent screen, phenomenon named later
on „Rutherford scattering" fig.13. As accelerated alpha particle source
he used a little oven with radioactive substance. The information supplied
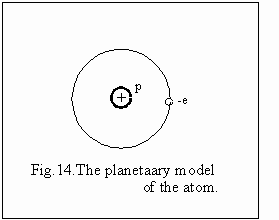
by this experiment allowed Rutherford to elaborate an atomic planetary
model fig.14, establishing also the main sizes. Under such a model appears
for the first time the notion of atomic nuclei, a very restricted area
in the centre of the atom, where it is situated almost the entire mass
of the atom and the positive charges. Similar to the planetary system
of the sun, the negative electric charges, the electrons rotate around
the nuclei, under the action of the electrostatic forces of attraction,
shown with certainty by the experiment, balanced by the centrifugal forces
of the electrons. The functioning of this model explains the magnetical,
optical and chemical properties of the atom, as phenomenon established
by the properties of the electronic shell.
-
His
publication in 1911 makes him being rapidly known by the physicians and
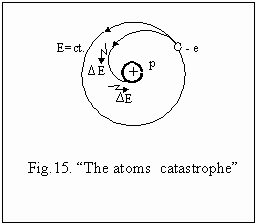
there appeared the first critics. On theoretical plan, the electron with
its negative charge, in his orbital movement, according to the electrodynamic
laws, should permanently radiate electromagnetic energy and at last, consuming
its energy, to fall in the nucleus. This remark remains in the history
of physics under the name of "the atoms’ catastrophe" fig.15. The way
it might be observed in fig.16, the model isn’t an exact copy of the solar
system, the atomic planetary model allowing any plan of the electronic
orbitals, while the planets rotate in the equatorial plan of the sun,
with less exceptions. Discussions and experiments have shown two possible
directions: Either was the model wrong or the laws of the electromagnetism
loose the validity at this level. The Danish physician Niels Bohr (1913)
solved this dilema, by using the quantic theory of Planck… Max Planck,
studying the spectral distribution and the laws of the radiation of heated
bodies, observed (1900) that the classic image of some oscillators that
continuously radiate energy does not suit to the real phenomenon and introduced
the idea of the quantification of these oscillators. Thus, the oscillators
that radiate and absorb radiations, might have only special energies,
that are multipes of the energy quantum E
= hn.
-
Bohr,
cooperator of the savant Rutherford, by using the most recent results
of the research in this field and by applying the theories introduced
by the quantic mechanics, where the absorbtion and emission phenomenon
of radiation are explained, formulated the following postulates: the electrons
move in the atoms on established, stable orbitals, whose energetical level
can’t vary continuously, but only discontinuously, quantified, being integer
multiple of h/2p. The movement of electrons
on orbits is done without any radiation und without energy absorption.
The trespassing of an electron from a stable orbital to another one is
done with radiation or absorption of energy, the energy of the radiated
or absorbed quantum being equal to the difference of the energies of the
two levels between that took place the electrons trespassing. According
to his postulates, Bohr analysis the condition of stability for the hydrogen
atom, having the nucleus formed of a proton with positive charge and an
electron that gravitates on a circle orbital under the coulomb attraction
between the positive nucleus and the negative electron, balanced by the
centrifugal force of the electron.
-
The
results obtained might not be applied to other atoms, imposing the need
of the models’ improvement.
-
THE
ATOMIC MODEL BOHR-SOMMERFELD
-
A.
Sommerfeld (1915), based on the quantic mechanics, brings as news the
atomic planetary model, the movement of the electron on elyptical orbitals,
the nuclei occupying one of the focuses of the elypse. This movement imposes
to the electron the permanent variation of the speed and mass, without
that the total energy being changed, phenomenon named "degeneration".
To this new term, there are added also others as "penetrating orbitals",
"fine structure of the spectra of hydrogen", etc.
-
"The
electronic orbitals" of the atomic model of Bohr-Sommerfeld doesn’t form
an exact description of reality, but gives the possibility of interpretation
for some phenomenon and behaviours of the atom investigated by spectroscopical
methods.
-
STUDIES
AND INTERPRETATION
-
The
spectroscopical analysis, by its exact results, shows the necessity of
improvement for the atomic model. The experimental study of the light
radiation, emphasizes the dual character wave-particle of the photon.
Louis de Broglie (1925) issues the hypothesis, later on acknowledged,
that the elementary particles demonstrate the wave-particle duality.
-
This
duality puts the theoretical physics in front of special difficulties,
because by applying the representation that exists in the classical physics,
in case of atomic processes, we came to the result that it looses its
validity.
-
L.
de Broglie going further on, conceives the material particle as a bundle
of waves "de Broglie" that do not spread into space.